The science behind calcium carbonate and ocean acidification
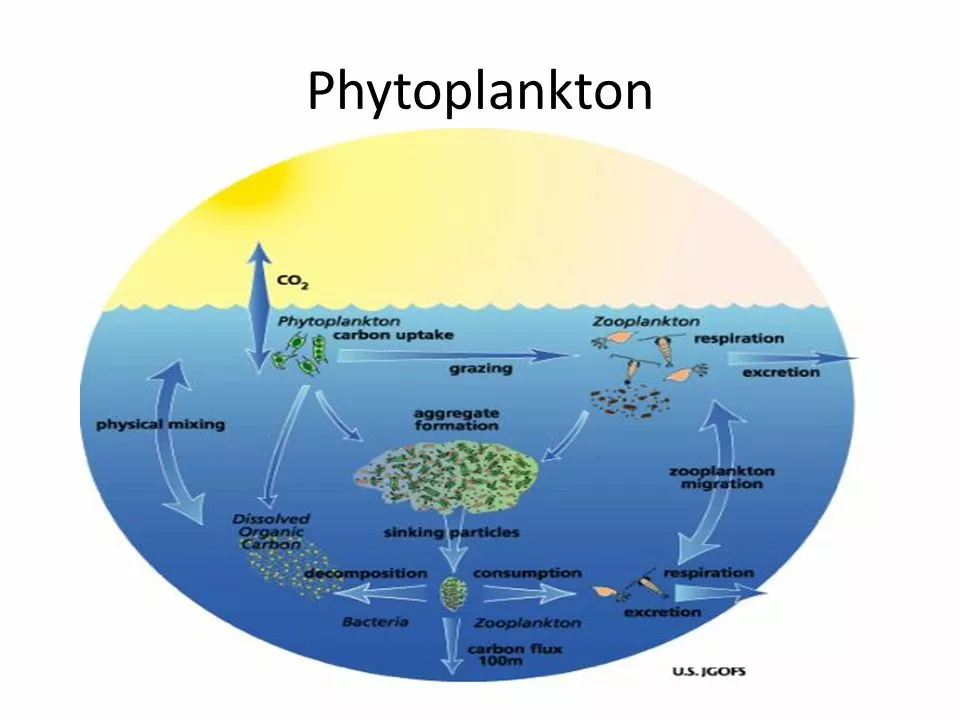
As a blogger, I've been researching the science behind calcium carbonate and ocean acidification, and it's fascinating! Ocean acidification occurs when carbon dioxide (CO2) dissolves into seawater, forming carbonic acid which then breaks down into bicarbonate ions, releasing hydrogen ions and increasing the ocean's acidity. This increase in acidity can have detrimental effects on marine life, particularly organisms that rely on calcium carbonate to build their shells or skeletons, like corals, mollusks, and some plankton species. Calcium carbonate is essential for these organisms, as it provides stability and protection, but ocean acidification reduces the availability of carbonate ions, making it more challenging for these creatures to build and maintain their structures. It's crucial that we continue to study and address the effects of ocean acidification on marine ecosystems to better understand and protect our ocean's biodiversity.