Understanding Ocean Acidification
Ocean acidification is a growing environmental issue that has captured the attention of scientists and environmentalists alike. It's caused by the uptake of carbon dioxide from the atmosphere, which leads to an increase in acidity levels in the ocean. In this article, we'll delve into the science behind calcium carbonate and ocean acidification, exploring its causes, impacts, and potential solutions.
The Chemistry of Carbon Dioxide and Seawater
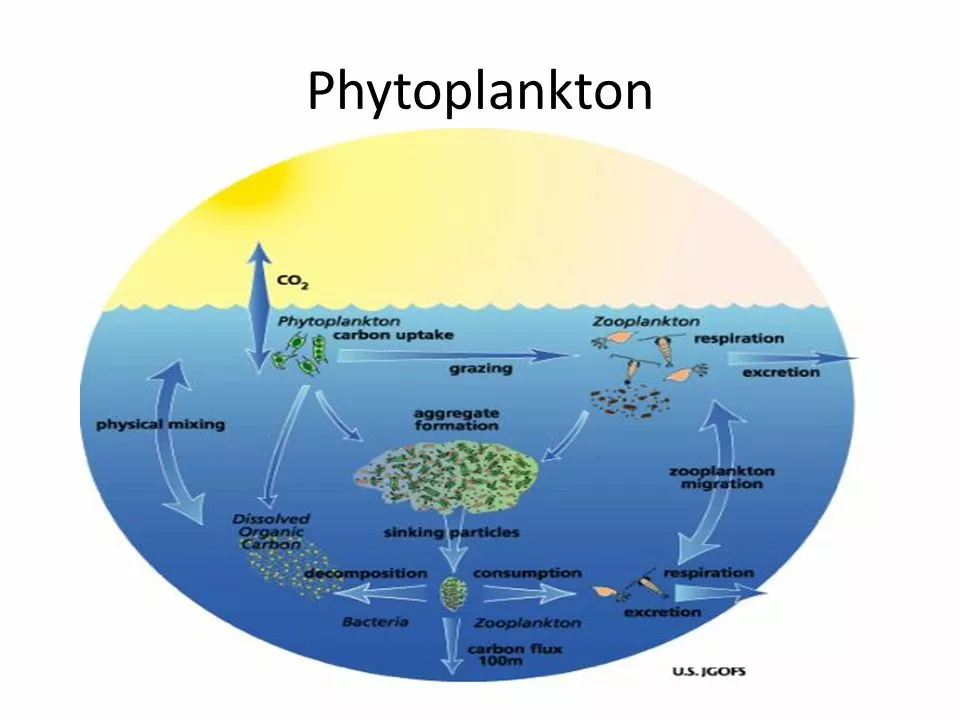
Before we can understand ocean acidification, we need to know a bit about the chemistry of carbon dioxide (CO2) and seawater. When CO2 dissolves in water, it forms carbonic acid (H2CO3). This acid then dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-). The increase in hydrogen ions leads to a decrease in pH levels, making the water more acidic. This process is the driving force behind ocean acidification.
Calcium Carbonate and Marine Life
Calcium carbonate (CaCO3) is a vital component of the shells and skeletons of many marine organisms, including corals, mollusks, and some plankton species. These organisms extract dissolved calcium (Ca2+) and carbonate ions (CO32-) from seawater to build their protective structures. However, as ocean acidity increases, the availability of carbonate ions decreases, making it more difficult for marine life to form and maintain their shells and skeletons.
The Carbonate Buffer System
The ocean's ability to regulate its acidity levels is primarily due to the carbonate buffer system. This system involves a series of chemical reactions between carbon dioxide, water, and carbonate minerals, which help to maintain a stable pH balance in the ocean. However, as more and more CO2 is absorbed from the atmosphere, the carbonate buffer system becomes less effective, leading to increased ocean acidification.
The Impact of Ocean Acidification on Coral Reefs
Coral reefs are among the most vulnerable ecosystems to ocean acidification. As pH levels decrease, the process of calcification – the formation of calcium carbonate structures – becomes more difficult for corals. This leads to weaker coral skeletons and slower growth rates, making it harder for reefs to recover from damage and increasing their susceptibility to disease and bleaching events.
Effects on Shellfish and Mollusks
Shellfish and mollusks, such as oysters, clams, and mussels, are also severely impacted by ocean acidification. As the availability of carbonate ions decreases, these organisms struggle to build and maintain their shells, leading to thinner and more fragile structures. This can result in increased mortality rates, reduced growth, and a decline in overall population numbers, which has significant implications for marine ecosystems and the human seafood industry.
Impacts on Plankton and the Marine Food Web
Many species of plankton, the microscopic organisms that form the base of the marine food web, also rely on calcium carbonate for their survival. Ocean acidification can affect the growth and reproduction of these crucial organisms, which can have cascading effects throughout the entire marine food web. This can lead to a decline in the overall productivity of the ocean and disrupt the balance of marine ecosystems.
Reducing CO2 Emissions to Combat Ocean Acidification
One of the most effective ways to address ocean acidification is to reduce global CO2 emissions. By transitioning to cleaner energy sources, promoting energy efficiency, and implementing carbon capture and storage technologies, we can help to slow down the rate of ocean acidification and give marine life a fighting chance to adapt to the changing conditions.
Monitoring and Research Efforts
Continued monitoring and research are essential for understanding the full extent of ocean acidification and its impacts on marine life. By collecting data on seawater chemistry, marine organism populations, and ecosystem health, scientists can develop a more comprehensive understanding of the issue and inform policy decisions aimed at mitigating its effects.
Adaptation and Resilience in Marine Ecosystems
While reducing CO2 emissions is crucial, it's also important to recognize that some level of ocean acidification is now inevitable. As a result, efforts must be made to support the adaptation and resilience of marine ecosystems. This can include protecting critical habitats, restoring damaged reefs, and supporting the development of more resilient species and strains that are better equipped to cope with changing ocean conditions.
In conclusion, the science behind calcium carbonate and ocean acidification is both fascinating and concerning. By understanding the chemistry at play and the impacts on marine life, we can take steps to mitigate the effects of this growing environmental problem and protect our oceans for future generations.





Craig Venn
The carbonate buffer system is key here. When CO2 dissolves, it shifts the equilibrium toward bicarbonate and H+, reducing carbonate ion concentration. That's why calcifying organisms are getting hammered. The pCO2 levels today are higher than in the last 3 million years. We're essentially running a massive geochemical experiment with no control group.
Amber Walker
This is wild I mean like seriously think about it corals are basically underwater skyscrapers made of rock and they're dissolving like sugar in tea
Matthew Williams
So let me get this straight we're blaming fossil fuels for fish having bad days now? Next they'll say my grill is sinking the Titanic.
Sarah Major
The science is clear but nobody wants to hear it. People still think the ocean is infinite and will just absorb everything. It's not a trash can. It's a living system and we're killing it slowly and call it progress.
Zach Harrison
I work with shellfish hatcheries. We've seen larval mortality spike 40% in the last 5 years. It's not theoretical. It's in our tanks. We're losing entire generations before they even settle. The industry is terrified.
Terri-Anne Whitehouse
Fascinating how the same people who scream about carbon footprints ignore that the ocean has been acidifying naturally for millennia. The real issue is anthropocentric panic disguised as science.
charmaine bull
I think we need to stop thinking of this as just a chemistry problem. It's a cultural one too. We treat the ocean like a resource bank instead of a relative. Maybe if we saw it as family we'd stop stealing from it.
Dave Collins
Ah yes the classic 'ocean acidification' narrative. Next you'll tell me the moon is made of cheese and the sun is just a very angry lightbulb.
Sue Ausderau
It's strange how we can map galaxies but struggle to protect the life in our own backyard. Maybe we're not as advanced as we think. Maybe we're just good at building things and bad at preserving them.
Torrlow Lebleu
You think this is bad? Wait till the pH hits 7.8. Then watch the entire food chain collapse. And don't even get me started on the economic fallout. This isn't climate change. This is a slow-motion extinction event.
M. Kyle Moseby
So we're supposed to stop using electricity so fish can have nice shells? That's dumb. Just make stronger shells. Nature adapts.
Nate Barker
All this doom talk. Meanwhile China's building coal plants faster than we can recycle soda cans. You're crying about coral while they're turning the Pacific into a soup.
Christine Mae Raquid
I knew this was coming. The ocean is crying. The whales are mourning. The plankton are whispering our names in the dark. We broke it. We broke everything.